Towards the understanding of synaptic functions to prevent age dependent memory impairment
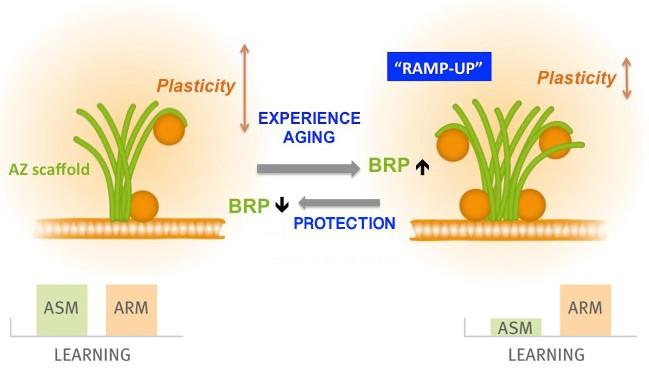
Plasticity model
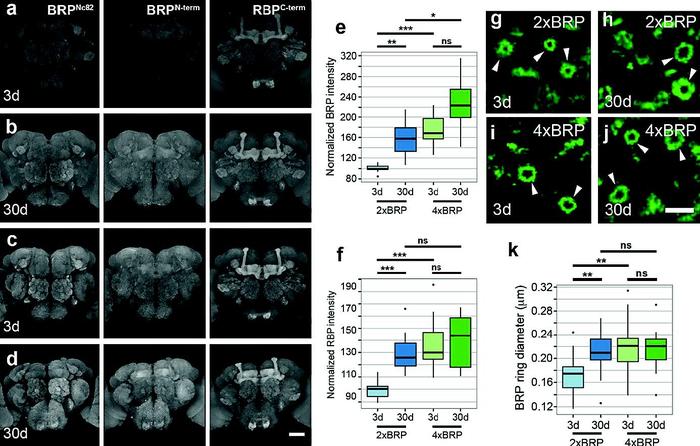
Ultrastructural, opto-physiological and behavioral analysis of synaptic organization within the aging Drosophila olfactory system.
All animals form memories to adapt their behavior in a context-dependent manner. With increasing age, however, forming new memories becomes less efficient, due to a largely unknown etiology. Searching for a mechanistic basis of age-induced memory impairment (AMI), we identified a brain-wide, age-associated upshift in the ultrastructural size and release function of presynaptic active zones (AZs) in the fruit fly Drosophila. This phenomenon we called “RAMP-UP” was absent from aged animals being pharmacologically protected from AMI, while genetically provoked RAMP-UP eliminated memory formation in young animals. Our data provoke the hypothesis that RAMP-UP narrows the synaptic plasticity space by adjusting the efficacy of memory formation to increasing lifetime, with the functional and structural AZ status operating a major “adjustment screw”. Based on our previous analysis of principal AZ organization, we here start by subjecting the AZ to a rigorous analysis concerning its role in olfactory memory formation. We then study the influence of aging and, finally, extend the analysis to the circuit level, using the recent advances in understanding learned behavioral processes in the olfactory system. My main aims are to:
- elucidate the role of short-term functional and longer term structural plasticity processes at AZs for forming new aversive olfactory memories;
- test directly whether AZ RAMP-UP interferes with plasticity processes encoding aversive memories; and
- analyze whether RAMP-UP operates locally or globally, and how information encoding and learning related processes intersect RAMP-UP throughout the olfactory system.
This “multiscale” analysis will enable me to generate a mechanistic biological model bridging the synaptic and the behavioral level. This model will also provide a basis to study information storage strategies in an aging and experience-forming brain.
Age-induced memory impairment: a biological and medical challenge
The first kiss, the smell of your mother’s cooking or your child’s first word, memories such as these are imprinted tightly into our brains and can be recollected even years later. However, the efficacy of forming new memories (“learning”) vanishes drastically for many of us as we grow older. This age-induced memory impairment (AMI) arose as one of the top public health threats during the past century. Our understanding of this process aims eventually at the delay and/or prevention of such age-related pathologies, with potential beneficial effects for health systems and later quality of life. The aging process itself, however, is subject to a complex interaction of regulatory and executory mechanisms. Consequently, the differentiation of causative from correlative or even adaptive and protective changes is a fundamental but still unsolved problem of studying cognitive aging [4]. Cognitive decline in humans typically starts in midlife and deepens with proceeding age, suggesting that age itself is the greatest risk factor. Thus, from a medical perspective, prevention of these pathologies ultimately necessitates a thorough understanding of the molecular mechanisms underlying their links with the aging process. Normal aging in rodents, nonhuman primates and humans seems to particularly affect the hippocampus and the dorsal prefrontal cortex, brain areas crucial for episodic memory and working memory, respectively. In these areas, AMI is, however, not linked to major neuronal cell death, as seen in neurodegenerative disorders. Therefore, if neuron numbers are not grossly altered, why do we develop memory deficits with aging? Investigations within brain regions with crucial roles in cognitive processing point towards age-related changes of synaptic structure and plasticity , for review see. However, causal relationships between these phenomena and AMI have not been established at this point in time. This grant will establish a biological model of AMI bridging the molecular status of identified, memory-forming synapses with the behavioral display of memory. Taken together, there is a need to understand the mechanistic basis of AMI deeper. This grant will establish causal relations directly between lifetime-associated synaptic changes and memory impairment, and deliver a conceptual frame for a mechanistic understanding of the phenomenon.
Modeling the synaptic basis of AMI in Drosophila
It is a challenge to study AMI mechanistically: Firstly, the long lifespan of many animal models makes tight experimental control difficult and, secondly, the problem of separating causal from epiphenomenal events makes it problematic. Consequently, being able to monitor physiological changes across the lifespan, as well as to effectively test age-associated changes for causality, will be pivotal to understanding the principles of AMI. Pavlovian olfactory conditioning in Drosophila is an established model for understanding the neural basis of associative learning. Flies learn to associate an odor with the negative reinforcement of mild, electric shock or with the positive reinforcement of a food reward. Such aversive or appetitive memory of this learned association is tested in a T-maze, in which trained flies avoid or approach the punished or rewarded odor, respectively. Genetic investigations of Drosophila have uncovered numerous genes whose products are now known to be essential for olfactory memory formation. Similar to vertebrates, the Drosophila brain uses population-level sensory coding, yet retains a simpler, more well-defined circuit organization in comparison to vertebrates.
Drosophila also offers a comparatively short lifespan (about 60 – 80 days) and superb, advanced genetics, and, thus, has been used successfully to model age- and lifetime-associated changes of memory formation. Aversive olfactory memories (in response to electric shocks) are subject to age-induced decline in Drosophila, as first shown by pioneering work by the Saitoe lab. In Drosophila, memory acquired after a single olfactory conditioning cycle can be separated into three distinct phases: short-, intermediate- and long-lasting memory. Intermediate term aversive olfactory memory can be further dissected into anesthesia-sensitive memory (ASM) and anesthesia-resistant memory (ARM) components, depending on their sensitivity versus amnestic cooling. Unlike ARM, which is stable with age, it is the ASM component which is strongly impaired with aging ). Here, AMI shows an onset at about 10 days of age and plateaus at about 30 days of age. As in other memory model systems, structural and functional changes at synapses (“synaptic plasticity”) are generally considered to underlie learning and memory in Drosophila as well. Similar to certain synaptic terminals of the mammalian brain, presynaptic plasticity processes are of pivotal importance for Drosophila olfactory learning and memory. Thus, a deep understanding of presynaptic release mechanisms is imperative in order to address the synaptic basis of memory formation and its age-induced impairments in this system.
In short, the organizational and cellular principles of forming olfactory memories show many similarities between Drosophila and mammals. The age-induced decline of aversive olfactory memories provides a powerful model system for scrutinizing molecular and cellular mechanisms provoking AMI in the Drosophila system.